The group of Florian H. Heidel's research interest is the development of myeloid neoplasia during aging of the hematopoietic system. The researchers employ genetically engineered mouse models and RNAi technology to functionally characterize signaling pathways and molecules involved in self-renewal, differentiation and aging of hematopoietic stem cells. Moreover, they aim to identify novel therapeutic targets for clinical therapy of aging associated myeloid cancers.
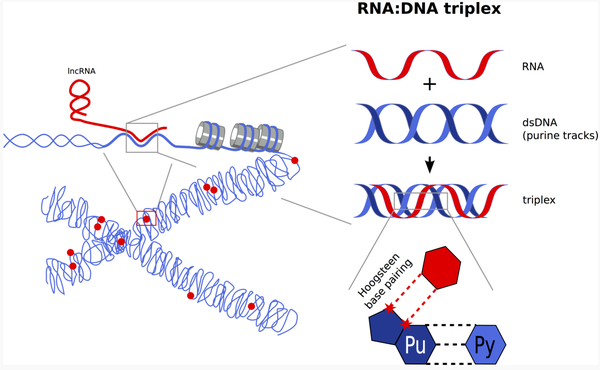
The group of Holger Bierhoff work aims to comprehensively identify and characterize ncRNA:DNA triplexes and their role in shaping the chromatin landscape. Chromatin is a complex of macromolecules that wraps DNA into a more compact shape, thus regulating gene expression and preventing DNA damage. Bierhoff's lab investigates how triplexes can compete with binding of proteins or with formation of alternative nucleic acid structures at the target sites, but also how (and which) chromatin-associated factors are recruited by triplexes. Together, the group hopes that their work will provide a profound understanding of ncRNA-mediated triplex-formation, which, according to the vast number of genomic sites with triplex-forming potential, is an important and abundant mechanism of epigenetic regulation.
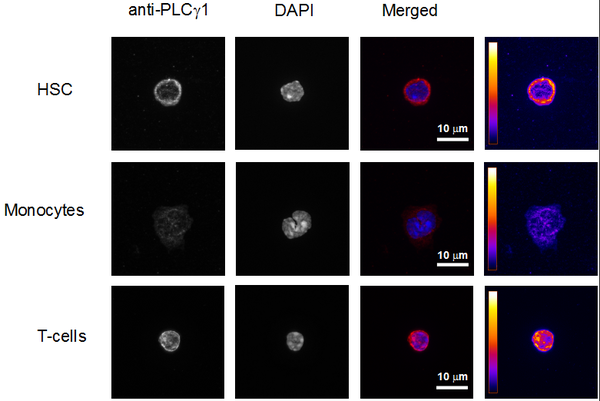
The group of Claudia Waskow works on hematopoietic stem cells (HSC). Stem cell maintenance is essential for continuous tissue formation during steady-state and under stress conditions such as aging. The constant supply of de novo generated mature cells from adult stem cells is pivotal for the lifelong function of many organs, in particular for tissues with high turn-over rates such as the gut, skin and blood. Understanding the mechanisms of fate choice in stem and progenitor cells holds the promise of replacement of tissues that lost their functionality during aging by engineered tissues in the future.
One of the most thoroughly studied adult stem cell types is the HSC that gives rise to all blood cells through a process called hematopoiesis. Continuous hematopoiesis is fundamental for a functional immune system that prevents the outbreak of infections and cancer formation. HSCs can be prospectively isolated to very high purity, and after bone marrow transplantation the infused donor HSCs disclose their amazing regenerative potential and continuously generate blood cells over long periods of time in the recipients.
Despite the precise phenotypic description of HSCs the molecular mechanisms, including signaling pathways and receptor interplay, underlying fate-choice decisions are not resolved. Failure of hematopoiesis can lead to life-threatening blood disorders disclosing the need for a tight regulation of fate choices in HSCs to ensure the welfare of the organism. Further, during aging, loss of HSC functionality leads to the weakening of immune system. We focus on cell-intrinsic and extrinsic niche-mediated signals that regulate HSC fate.





